Lecanemab Alzheimer’s treatment
A Breakthrough in Alzheimer’s Care
Alzheimer’s disease progresses gradually and leads to memory and thinking difficulties. Although there is no cure yet, new medications like lecanemab provide hope. In 2023, the FDA approved lecanemab (sold under the brand name Leqembi) for patients in the early stages of Alzheimer’s. The drug directly targets the root cause of the disease and helps delay its development.
What Is Lecanemab?
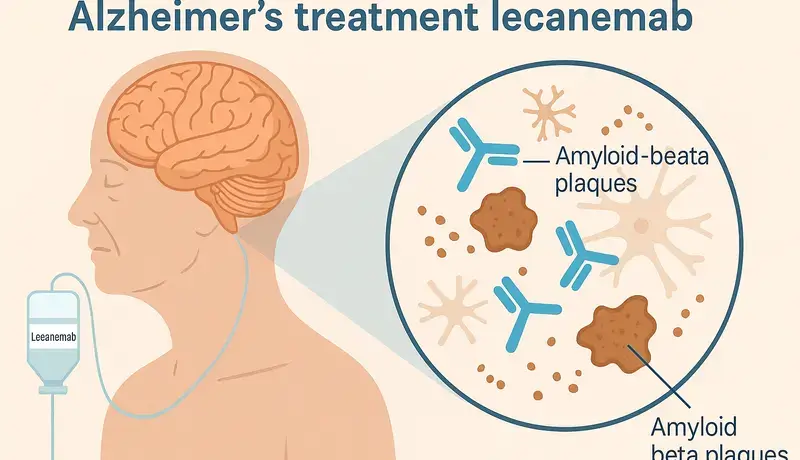
Lecanemab is a monoclonal antibody created in a lab to act like a natural immune protein. It binds to amyloid-beta plaques in the brain and helps remove them. These plaques are known to speed up Alzheimer’s disease, so clearing them can slow down cognitive decline.
How Effective Is It?
The Clarity AD clinical trial showed that patients who received lecanemab for 18 months experienced 25–30% slower mental decline than those who didn’t receive treatment. While lecanemab did not stop memory loss, it clearly delayed the progression.
Furthermore, brain scans revealed that the medication helped reduce or clear amyloid buildup, confirming its biological impact.
Does It Have Side Effects?
As with all medications, lecanemab may cause some side effects. The most serious risk is a condition called Amyloid-Related Imaging Abnormalities (ARIA). ARIA includes swelling (ARIA-E) and minor brain bleeding (ARIA-H), visible on MRI scans. Fortunately, these effects remain uncommon.
In the Clarity AD trial, less than 1% of participants experienced each type of ARIA. These results suggest that lecanemab is generally well tolerated.
Real-World Data Confirms Safety
A recent study from the Washington University Memory Diagnostic Center analyzed the treatment outcomes of 234 patients with early Alzheimer’s. These individuals received lecanemab in a real-world clinic setting rather than in a tightly controlled trial. The findings support the drug’s safety and effectiveness.
- Only 1.8% of people in the earliest stage of Alzheimer’s showed signs of ARIA.
- Among those with mild Alzheimer’s, 27% developed ARIA, but symptoms faded after a few months.
- No patients died or faced severe complications.
Expert Opinions
Dr. Barbara Joy Snider, co-lead author of the study, emphasized the encouraging results. She noted that the outcomes closely matched those of the clinical trial. According to her, this consistency proves that clinics can use lecanemab safely under proper monitoring.
She also added, “Our experience reassures us. We can now offer this treatment to suitable patients with confidence. Still, we will keep monitoring side effects and memory decline over time.”
A Step Forward for Alzheimer’s Patients
Lecanemab stands out as a milestone in the treatment of Alzheimer’s. Even though it does not cure the disease, it offers patients more time with better brain function. The latest study shows that its side effects remain manageable and rare. Most importantly, it gives doctors a safe and effective option for treating early-stage cases.
Researchers plan to continue tracking patients and studying newer drug combinations. But for now, lecanemab represents real progress in the fight against Alzheimer’s.
Read more here: Lecanemab drug slows Alzheimer’s: Safe, effective, side effects, real-world data
Lecanemab Alzheimer’s treatment